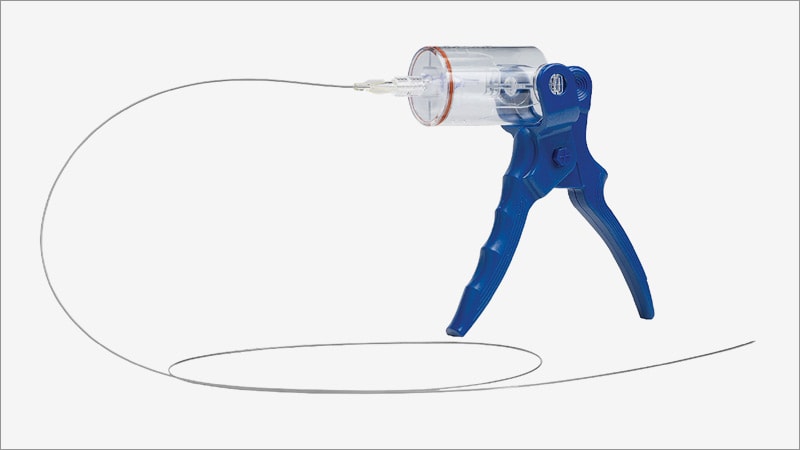
The US Food and Drug Administration (FDA) has approved the Aspire MAX 7–11F mechanical thrombectomy system to remove blood clots from peripheral vessels, Control Medical Technology, the company that makes the system, has announced.
"The Aspire MAX 7–11F Mechanical Thrombectomy System includes (20) new large-lumen, flexible, and kink-resistant catheters with dilators powered by the Aspire aspirator and/or an electromechanical pump," company President Shawn Fojtik said a statement.
The Aspire MAX 7–11F mechanical thrombectomy system includes 7F (0.090-inch) outer diameter (OD) to 11F (0.140-inch) OD catheters with flexible dilators for improved tracking. Catheters may be connected to the Aspire aspirator and/or an electromechanical pump for increased speed, force, volume, and control, the company says.
Control Medical's system also includes the Aspire MAX 5–6F mechanical thrombectomy system with over-the-wire catheters for peripheral vasculature and the Aspire RX-LP mechanical thrombectomy system with rapid exchange catheters for peripheral and coronary vasculature. More information is available online.
"Blood clots range from soft-fresh clots to hard-aged thrombus. Clinicians need more cost-effective tools to remove blood clots. We plan to introduce more catheter and electromechanical pump innovations for use in peripheral, coronary, and neurovascular procedures," Fojtik said.
For more Medscape Neurology news, join us on Facebook and Twitter
"Max" - Google News
May 08, 2020 at 09:41PM
https://ift.tt/2WdKnJJ
FDA OKs New Aspire MAX Mechanical Thrombectomy System - Medscape
"Max" - Google News
https://ift.tt/2YlVjXi
Bagikan Berita Ini














0 Response to "FDA OKs New Aspire MAX Mechanical Thrombectomy System - Medscape"
Post a Comment